Source: Link Testing Instruments Co., Ltd
Infusion bags are widely used in clinical treatment with which the medicine can be directly applied to the venous blood increasing therapeutic effect. There are two main categories of infusion bags: PVC and Non-PVC. Today most of the non-PVC infusion bags are made of multi-layer copolymerized compound membrane, which contains PP, PE, PA and SEBS, etc. Non-PVC is the safest material of infusion bag by now. It is free of plasticizer, which doesn’t adsorb or react with the liquid medicine. In addition, non-PVC infusion bag is featured with low temperature resistance and alkali separation problem with the glass bottle will not happen to non-PVC infusion bags.
In order to ensure the safety of the infusion, the closure of the infusion bag shall have proper puncture resistance so that insoluble particle will not generated during puncturing process. Generally, the possibility of generating insoluble particles is inversely proportional to the number of puncture attempts. In other words, the closure should be easy to puncture through so that particles that may enter the liquid medicine can be minimized. On the other hand, the puncture resistance of the rubber closure cannot be too low, otherwise, it may be punctured through during the storage or transportation process. Therefore, it is necessary to measure the puncture resistance of the rubber closure of infusion bag.
This article provides a simplified test method for the puncture resistance test of rubber closure of infusion bag for reference, which may be helpful to the manufacturers and users of infusion bags. The testing instrument used for the puncture test is Link Testing’s LTS-05 Medical Packaging Tensile Tester.

Figure 1. Multi-layer Co-extruded Infusion Bag with Rubber Closure
The tests shall be performed according to the following procedures.
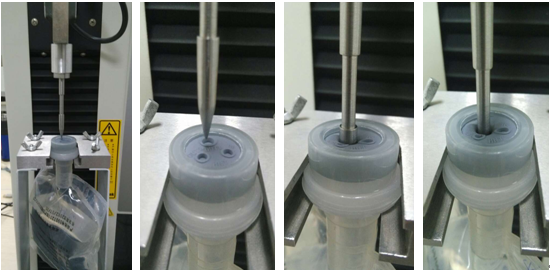
Figure 2. Testing Process
(6) Repeat the procedures of step 3 to step 5 until all the specimen tests are completed.
The puncture resistance of the rubber closure of infusion bag shall conform to certain standards. According to the test results obtained in the tests mentioned above, the infusion bag manufacturers can determine whether their products can meet the requirements for actual use.
For more details about Link Testing’s LTS-05 Medical Packaging Tester, please visit www.linktesting.org
About Link Testing Instruments Co., Ltd:
Link Testing Instruments Co., Ltd is one leading supplier of packaging testing instruments, which is headquartered in Jinan, China.